Thalidomide acts by a variety of mechanisms; The treatment, called abecma, was recently approved by the u.s.

Multiple Myeloma The Patient Journey The Biotechnologist
“nevertheless, we continue to add new drugs and new treatment regimens to push that moment patients run out of options further.
Newest treatment for multiple myeloma. Thankfully, the new immune therapies, such as daratumumab (darzalex®), attack antigens expressed on myeloma cells irrespective of these diverse dna mutations, thus providing a broadly effective treatment approach. Food and drug administration (fda). Belantamab mafodotin, or belantamab for short, is an immunotherapy option for patients with multiple myeloma.
There are different multiple myeloma treatment options. Here are a few quotes i heard while attending the session a glimpse into the future of myeloma patient management presented by dr. Amrita krishnan at ash 2021.
Ohc doctors offer a new treatment option for patients with multiple myeloma. Its efficacy is well known in. Researchers at karolinska institutet have investigated the use of low dose venetoclax, an experimental drug, for the treatment of the heterogeneous cancer disease multiple myeloma in patients who.
Selinexor is a new type of multiple myeloma drug called a selective inhibitor of nuclear export (sine). Multiple myeloma is a type of blood cancer for which there is no cure. Food and drug administration (fda) today announced approval of daratumumab plus hyaluronidase (darzalex faspro®) in combination with pomalidomide and dexamethasone (pd) for the treatment of adults with multiple myeloma who have received at.
New treatment indication for multiple myeloma drug. After decades of minimal progress, two new classes of drugs with novels mechanisms of action: The food and drug administration (fda) has approved three new drugs for the treatment of multiple myeloma that has returned after prior therapy.
B cell maturation antigen (bcma) is a validated therapeutic target for mm, as it is expressed at high levels on the type of b cells that cause this malignancy. Immunomodulatory drugs (thalidomide and lenalidomide) and proteasome inhibitors (bortezomib) have shown great activity for the treatment of multiple myeloma. Most will experience relapses over several years, and with each relapse, treatment becomes less effective.
A stem cell transplant may be part of treatment. Minimal residual disease minimal residual disease is a term used when tiny amounts of myeloma cancer cells are still present in the bone marrow after treatment. In 2020, of all patients newly diagnosed with a blood cancer, 18% are expected to be diagnosed with this type of blood cancer.
Food and drug administration (fda) recently granted the seventh approval to daratumumab for the treatment of multiple myeloma. It's taken with pomalidomide and dexamethasone, and is in the same group of medicines as daratumumab. “kyprolis is the new velcade.”
This injectable antibody is approved alone or in combination with other drugs at different states of multiple myeloma treatment. New research is showing that by treating these patients sooner than waiting for symptoms may delay when active myeloma starts and may also improve survival. Patients with multiple myeloma also receive supportive treatments, such as transfusions to treat low blood cell counts, and antibiotics and sometimes intravenous immunoglobulin (ivig) for infections.
The latest standard of care for newly diagnosed multiple myeloma patients the combination of a proteasome inhibitor and an immunomodulatory agent plus the steroid dexamethasone is the standard of care for newly diagnosed patients. Treatments of multiple myeloma can help relieve pain, control complications of the disease, stabilize the body and slow the progress of the cancer. Isatuximab is a new antibody treatment that is used to treat multiple myeloma in adults where 3 other treatments have not worked.
Yolanda brunson led a discussion with prominent researchers to ask about the latest multiple myeloma treatment options: Depending on the stage, the average survival rate is five to seven years. Teclistamab is a bispecific antibody.
Fda approves new treatment for refractory multiple myeloma. Belantamab mafodotin, daratumumab (darzalex) and selinexor (xpovio). Proteasome inhibitors, the therapeutic backbone of.
Patients being treated for multiple myeloma have a promising new option for treatment. Approach to the treatment of relapsed multiple myeloma in first relapse (a) and second or higher relapse (b). The fda approved it for treatment of.
This may mean the average multiple myeloma patient can expect to learn about some new treatments, names of new meds and new tests. The main treatments for multiple myeloma include: Current knowledge and key points:
In the past 20 years, few diseases have seen as great progress in their treatment as multiple myeloma. Getting a bone marrow transplant often. Although several effective treatments exist for multiple myeloma, patients with this blood cancer face a disheartening reality:
On march 26, the food and drug administration (fda) approved idecabtagene vicleucel (abecma) for people with multiple myeloma that has not responded to or has returned after at least four different prior cancer treatments. On november 16, the fda approved daratumumab (darzalex®) for patients who have previously received at least three prior
The laser beam penetrates the skin and acts to promote the formation of osteocytes,. New horizons in treatment of osteoporosis daru.
En español | a promising new drug for treating osteoporosis in postmenopausal women at high risk of bone fractures is expected to be available as soon as next week.
Newest treatment for osteoporosis. Osteoporosis is a condition where there is a thinning of bones and they become brittle and fragile leading to increased risk of fractures, cracks, and breaks compared to normal bones. Several factors influence bone health, but the activity of bone cells is one of the most critical components to understanding this disease. Evenity (romosozumab) is a type of therapy known as a monoclonal antibody, and it helps build new bone by blocking the effect of a protein called sclerostin, the agency explained.
New avenues to osteoporosis therapy most current osteoporosis therapies include the use of bisphosphonates, which block osteoclast activity. Bioflex pakistan new treatment for osteoporosis. The goal of pharmacological therapy is to reduce the risk of fractures.
A potential increased risk of blood clots; Osteocytes are living cells that are formed once osteoblasts are buried in the bone matrix. The royal osteoporosis society (ros) has welcomed the decision by the scottish medicines consortium (smc) to approve the use of romosozumab in nhs scotland for the treatment of women with severe osteoporosis who have sustained fractures and are at high risk of further broken bones.
Approximately 10 million americans have osteoporosis, according to the national osteoporosis foundation. Osteoporosis (op) is a chronic bone disease characterized by aberrant microstructure and macrostructure of bone, leading to reduced bone mass and increased risk of fragile fractures. A newer treatment option could soon become available for women with osteoporosis.
Treating osteoporosis and rheumatoid arthritis encouragingly, the researchers were able to prevent bone loss in lab mice by blocking elmo1, including in two different models of rheumatoid arthritis. Parathyroid hormone is produced naturally in the body. It's taken as a daily tablet.
New drug treatment for osteoporosis approved in scotland. More than 30 years of experience. Researchers at purdue created a prodrug form of the.
Food and drug administration (fda) approved the new medication romosozumab (brand name evenity), from the drugmaker amgen, which appears to dramatically boost bone density. Zoledronic acid (reclast), an annual iv infusion. Amgen has set the u.s.
Read more about raloxifene for treating osteoporosis. Romosozumab was originally reviewed and denied approval by the united states food and drug administration (fda) due to safety concerns, but the manufacturers have recently resubmitted a new approval application with additional. Risedronate (actonel), a weekly or monthly pill.
New treatments for osteoporosis may offer advantages over existing oral bisphosphonates, particularly regarding compliance. Bone cell activity points to new osteoporosis treatment. Alendronate (fosamax), a weekly pill.
Osteoporosis affects 10 million people in the united states, the majority of them women. Raloxifene is the only type of serm available for treating osteoporosis. Researchers at purdue created a prodrug form of.
The study is the first that compares the effect of two osteoporosis medicines on fractures. It occurs when less calcium is deposited in the bone and more is taken out from the bones. Ibandronate (boniva), a monthly pill or quarterly intravenous (iv) infusion.
That's a drug that's currently in clinical trials. Side effects associated with raloxifene include: Purdue university innovators developed a stabilized form of human calcitonin, which is a peptide drug already used for people with osteoporosis.
Bisphosphonates are usually the first choice for osteoporosis treatment. Romosozumab is a new type of medication for treating osteoporosis that offers another treatment option for some women after menopause. Antiresorptive medications primarily decrease the rate of bone resorption while.
This leads to honeycombing of the bones leading to reduced bone density. Ad attending on time could avoid a surgery. If osteoporosis therapy is needed after the 12 doses, patients should begin an osteoporosis treatment that reduces bone breakdown, according to the fda release.
Purdue university innovators developed a stabilized form of human calcitonin, which is a peptide drug already used for people with osteoporosis. A new treatment for osteoporosis provides major improvements in bone density and more effective protection against fractures than the current standard treatment. Most current osteoporosis therapies include the use of bisphosphonates, which block the activity of bone resorbing cells, and thus prevent excessive bone resorption.
Lllt is a relatively new treatment for osteoporosis that’s promising because it has been proven to increase the production of osteocytes. These are the findings of a study published in the new england journal of medicine (nejm).
Steroid creams or ointments (topical corticosteroids) are commonly used to treat mild to moderate psoriasis in most areas of the body. Following two phase 3 clinical studies which demonstrated efficacy and safety, the u.s.
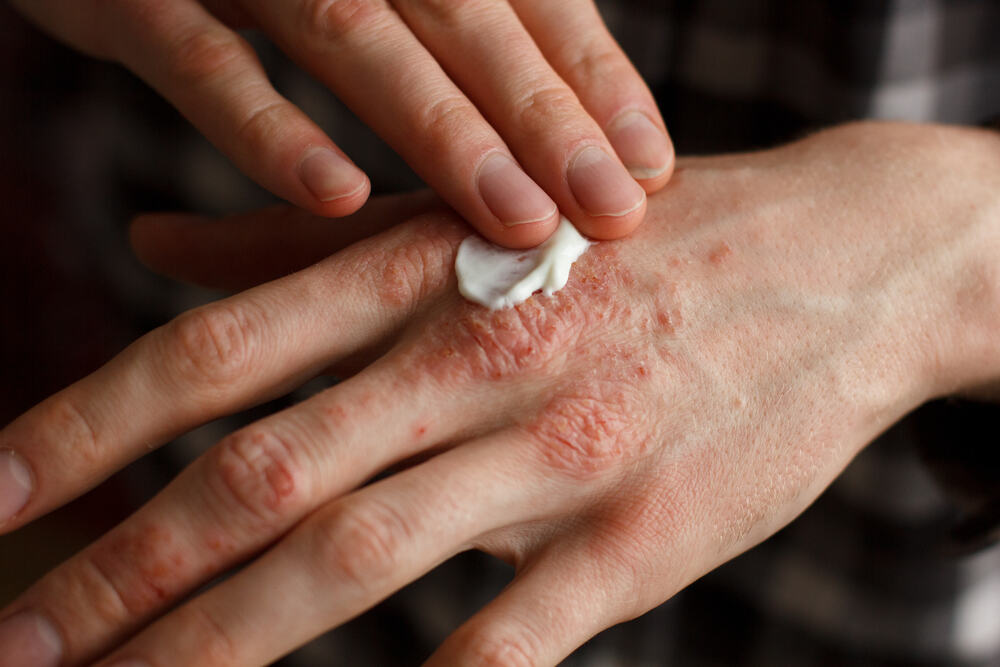
Benvitimod cream a new topical treatment for plaque psoriasis
This time it's back straight away and i'm wondering what.
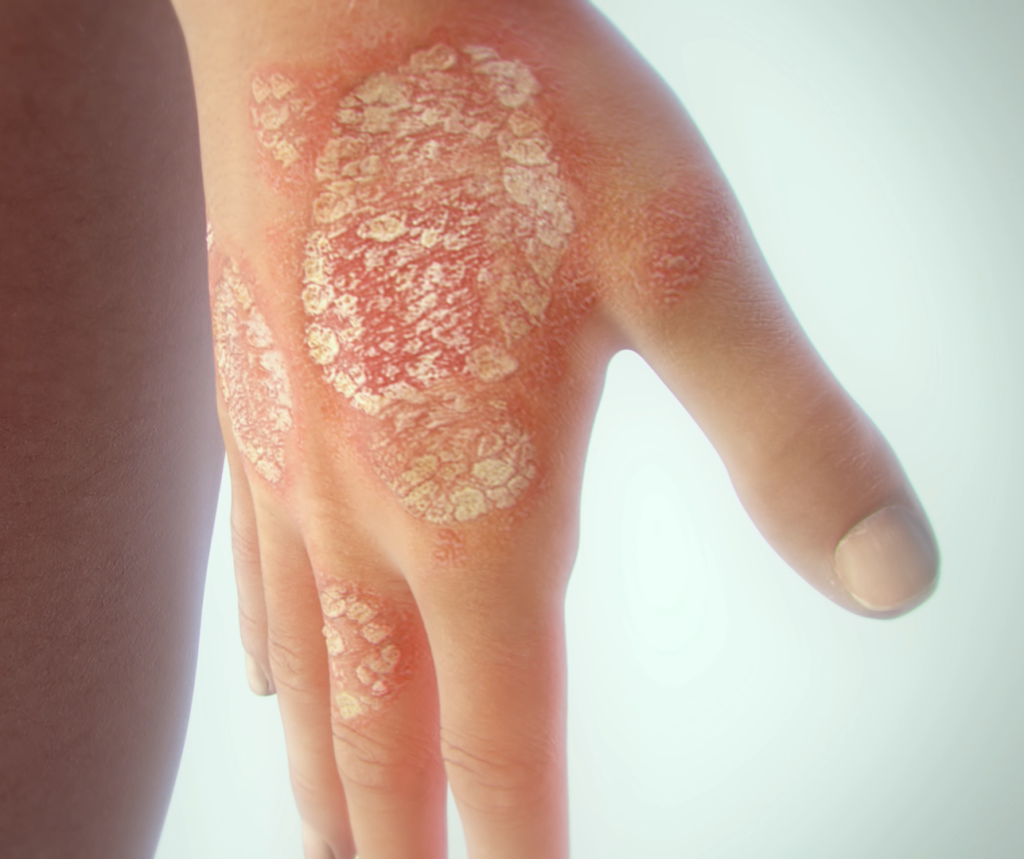
Newest treatment for psoriasis. Since then, we have had 14 systemic treatments approved for psoriasis; Psoriasis is a disease that can affect many aspects of professional and social life. The fda approved certolizumab pegol (cimzia) as a psoriasis treatment in may 2018.
This document incorporates and summarises information from a number of publications, including guidelines published by the national institute for health and care excellence (nice) [1,4], uptodate [2,7,10,16,17,23,26] and the nz formulary [20,22]. Biologics they're medicines made from substances found in living things. Painful blisters, but it is not contagious, and there is no approved treatment.
For psoriasis patients, try a new drug that improves symptoms and avoids complications. Fda approves upadacitinib for the treatment of adults with active psoriatic arthritis. Which is the best treatment for psoriasis in 2022?
New treatments for severe psoriasis. Currently, several treatments are available to help control psoriasis such as methotrexate, ciclosporin, and oral retinoids. The newest drugs for the treatment of plaque psoriasis.
At welling clinic, we have developed homeopathic formula, to help you recover faster and surely from psoriasis. Compared to coal tar or anthralin, the use of salicylic acid is more recent but is still one of the oldest treatments for psoriasis. However, the available treatments are.
Hi all, i've just finished a course of uvb treatment, have had 5 courses over the space of 10 years and it completely clears my skin up for around a year until it gradually comes back (i'm covered head to toe). Three per cent of the world’s population suffer from the skin condition known as psoriasis. The year was 1997 and the drug was tazarotene gel.
Psoriasis treatment usually starts with topical (applied to the skin) treatments, which can come in different formulations (creams, ointments, gels, etc) and have different active ingredients. Homeopathy has always been the best treatment for psoriasis if you want to cure psoriasis permanently and prevent complications of psoriasis. In this guideline, psoriasis refers to.
If these don’t prove effective, patients may then. Most people with psoriasis start their treatment under the guidance of a general practitioner (gp). The research council of norway summary:
Although incurable, new studies suggest that pycnogenol, the antioxidant extract of pine bark, can be a strong accompaniment to usual therapy. They frequently come to their physician asking if they are eligible for any of these agents and, if. The treatment works by reducing inflammation.
It had previously been approved to treat conditions. Though the biologics are perhaps the most promising of available psoriasis treatments, the decision to institute a given therapy may be fraught with complexity for the clinician. Currently, several treatments are available to help control psoriasis such as methotrexate, ciclosporin, and.
However, there has been virtually no innovation in topical therapy beyond New biologic therapies work well to treat psoriasis, and other new treatments are close to fda approval. 13 rows official answer.
Bimekizumab also has been shown to effectively treat psoriatic arthritis, a condition that affects 1 in 3 people with psoriasis, lebwohl said. It is relevant to the treatment of psoriasis in new zealand. The big news in topical agents is tapinarof, which is in development and does not contain any steroids, lebwohl says.
This slows the production of skin cells and reduces itching. Patients now hear of these promising new treatments for psoriasis via print, television and radio advertising; And then, more recent topical treatments for psoriasis include calcipotriol, calcipotriol / betamethasone, and tazarotene.
For psoriasis patients, try a new drug that improves symptoms and avoids complications. Painful blisters, but it is not contagious, and there is no approved treatment for it, and medicine seeks to find a treatment to solve its symptoms and avoid exposure. Food and drug administration (fda) has approved rinvoq® (upadacitinib) for the treatment of adults with active psoriatic.
Psoriasis is a disease that can affect many aspects of professional and social life. Posted fri 29 oct 2021 11.27 by smitch. For psoriasis to develop a new therapeutic option.
The regulator authorised the drug.